Energies and Forces – Introduction
Energy and force are two of the great unifying themes of physics and chemistry. But these two key concepts are crucial for the study of living organisms as well. In this chapter, we use a series of case studies to give a feeling for both the energy and force scales that are relevant in cell biology.
In the first part of the chapter, we consider some of the key energy currencies in living organisms, what sets their scale and what such energy is used for. One overarching idea is that the fundamental unit of energy in physical biology is set by the energy of thermal motions, namely, kBT, where kB is the celebrated Boltzmann constant and T is the temperature in degrees Kelvin. Our discussion of thermal energy centers on the way in which many biological processes reflect a competition between the entropy and the energy, a reminder that free energy is written as G=H-TS, where H is the enthalpy and S is the entropy. Whether we think of the spontaneous assembly of capsid proteins into viruses or the binding of chemoattractant to a chemoreceptor, the competing influences of entropy and energy determine the state of the system. Like everyone else, we then acknowledge the primacy of ATP as the energy currency of the cell. This discussion is followed by an examination of two of the other key energy currencies, namely, the storage of energy in transmembrane potentials and the origins of reducing power in compounds such as NADPH. We then turn to the study of the redox potential and the amazing series of molecular partnerships that have been struck in the oxidation-reduction reactions in the cell.
In the second part of the chapter, we complement our studies of energy by exploring the way in which energy is converted into useful work through the application of forces. Our study of forces begins by considering how both molecular motors and cytoskeletal filaments exert forces in processes ranging from vesicle transport to chromosome segregation to cell division to the motion of cells across surfaces. This is followed by a discussion of the physical limits of force-generating structures such as cytoskeletal filaments. How much force can an actin filament or a microtubule support before it will rupture?
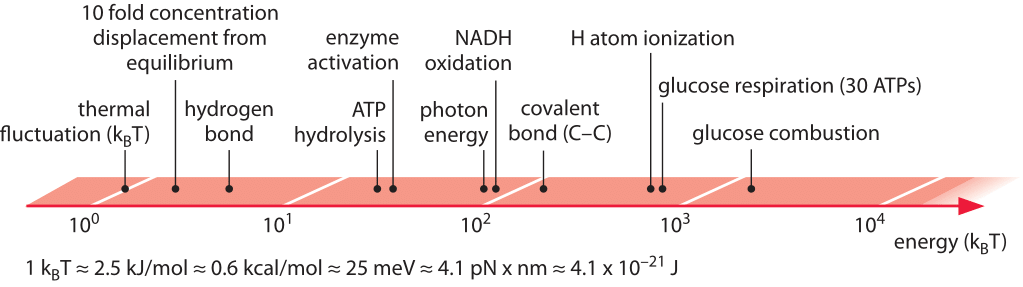
Figure 1: Range of characteristic energies central to biological processes. Energies range from thermal fluctuations to combustion of the potent glucose molecule. In glucose respiration we refer to the energy in the hydrolysis of the 30 ATP that are formed during respiration of glucose.
In working on writing this chapter it became apparent to us that some of the energies like those of a photon or combustion of a sugar are easy to pinpoint accurately. Others are trickier because they depend upon the concentration of the various molecular players such as the case of the hydrolysis of ATP. Finally there are the cases where it is very hard to even define, never mind providing a concrete value. Examples of these subtle cases include the energy of a hydrogen bond, the free energies associated with the hydrophobic effect or the entropic cost of forming a complex of two molecules. While it is easy to clearly define and separate the length of a biological object from its width it is much harder to separate say the energy arising from a hydrogen bond from the other interactions such as those with the surrounding water. Together, the case studies presented in this chapter acknowledge the importance of energy in biological systems and attempt to give a feeling for energy transformations that are necessary for cell growth and survival.

